Food processors must follow an integrated approach in order to prevent proliferation of listeria in the final product. Control of L. monocytogenes is required at all stages in the food chain.
The consumption of contaminated food is the main route of transmission of listeriosis, however because listeriosis cases are sporadic it is particularly difficult to detect links between human cases and causative foods. It is estimated that levels of L. monocytogenes below 100cfu/g of food represent a very low risk of listeriosis for all population groups.
Foods most often associated with human listeriosis include those which:
- Support the growth of L. monocytogenes
- Have a long shelf-life under refrigeration
- Are consumed without further listericidal treatments, i.e. treatments which would kill L. monocytogenes, e.g. cooking
Persistence in food processing environments is considered to be the major source of ready-to-eat ( RTE) food contamination. Persistence appears to be the result of (1) improper hygiene conditions and (2) high adaptive ability of listeria against physical–chemical factors, for example, biofilm-forming capacity.
The challenges for controlling L. monocytogenes are considerable given:
- It is widely present in nature and normally present in agricultural products
- Its high resistance to up to 20% salt and acidic pH
- Its ability to grow and survive at or below normal refrigeration temperatures to significant numbers when given sufficient time
- Its ability to grow quickly at ambient temperature (yet it does not survive pasteurisation)
- Its ability to form strong biofilms, which can be difficult to remove. Biofilms are likely to form in difficult to clean areas within processing environments
There are a number of contamination routes whereby L. monocytogenes can enter the food chain (Fig 1).
The following general recommendations apply to all sectors of the food chain:
- Implementation of GHP and Good Manufacturing Practices (GMP)
- Implementation of a food safety management system based on the principles of HACCP
- Testing against microbiological criteria
In addition: It is essential to conduct environmental monitoring to identify areas where L. monocytogenes proliferates.
Product testing in Listeria control
Role of indicator testing:
Testing in the environment and or finished product indicates that sanitation and zoning controls are working and that the environment is clean. Does not replace pathogen monitoring, it provides additional information. Verifying that a processing environment is under control requires independent testing for Listeria spp.
Role of finished product testing:
Does not ensure food safety! Focus on preventative controls and employ a pathogen environmental monitoring program to verify the effectiveness of the controls. Act on positive results!
Why is product testing alone not effective?
- Listeria is unevenly distributed within contaminated product and pockets of contamination may be missed
- Listeria are present in very low concentrations and grow slowly at refrigerated temperatures.
- Cross-contamination occurrences are sporadic
Primary sources of Listeria:
- soil and water for transmission to plant material, feed, animals and the food chain
- farm environment is an important natural source for raw material contamination.
Important to consider
- Higher pathogen concentration in raw material needs more effective control processes = to reduce concentrations to acceptable levels
- Increasing concentration of L. monocytogenes in the raw material entering the process = Increases (1) potential for contamination + (2) persistence in the processing environment
Food processing
- Most food processing steps have the potential to reduce pathogen loads through microbial inactivation or inhibition of growth
- Pathogen may survive mild process (i.e. washing) while more intense or severe processes (i.e. sufficient heating) may eliminate the pathogen
Re-contamination during further processing/handling:
- Increased handling = higher probability of contamination
- Sources of contamination = food contact surfaces, processing machinery and workers
Most important occasions for contamination
Contamination after heat processing + during further handling (cutting, slicing). Due to formation of biofilms, which may result in enhanced resistance to sanitisers, disinfectants and antimicrobial agents
Storage and consumer
- Packaging atmosphere, under vacuum or modified atmosphere conditions, can affect the growth of the pathogen during storage
- Use-by date affects growth of monocytogenes, since it is linked to the storage time of the product. Consumer understanding of use-by dates is often lacking and in practice adherence to these may be very variable.
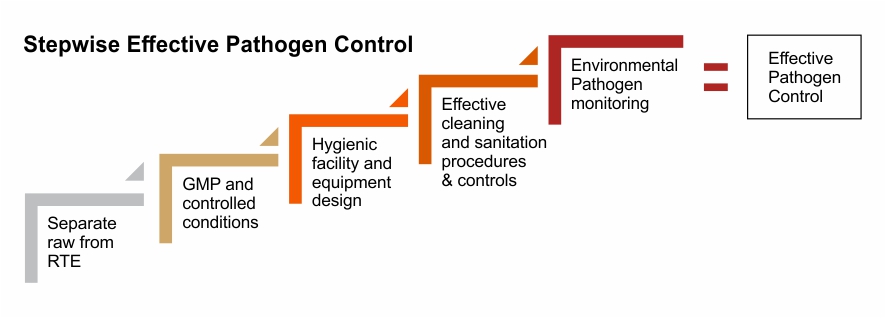
A Food Pathogen Control Program can lead to the food processor determining where to focus resources and how to create a plan for remediation.
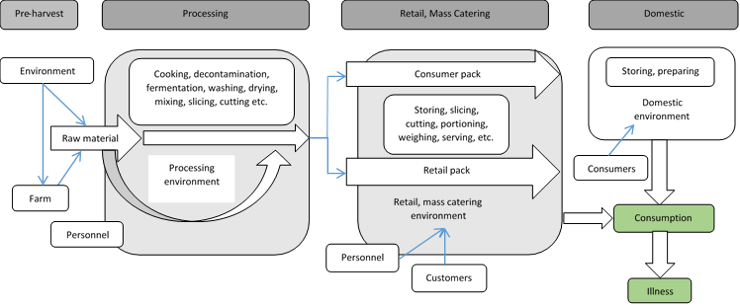
Figure 1: Transmission routes of Listeria monocytogenes in ready-to-eat (RTE) foods
About the Author
Professor Elna Buys runs the Department of Consumer and Food Science, University of Pretoria
She can be contacted at: elna.buys@up.ac.za
References
EFSA 2017, Listeria monocytogenes contamination of ready-to-eat 1 foods and the risk for human health in the EU (Draft), EFSA Journal
Food Safety Authority Ireland, 2011. Microbial fact sheet: Listeria monocytogenes, issue 1 September 2011
Innovation Center for US Dairy, 2015. Control of Listeria monocytogenes
Attorney Janusz Luterek, addresses questions on the legal implications of the listeria outbreak for the food industry...
The National Institute for Communicable Diseases (NICD) is reporting a very large outbreak of Listeriosis in South Africa. Here are some facts that you should know.
...