In the third part of this series, Chris explains process management. This is an area where many food companies go wrong as process management is much more than production processes. How to define the process, how to develop the top level of documentation, what can you exclude from ISO 9001 and how to control your documents are all covered in this final chapter of Starting the journey to ISO 9001 Certification.
Define your ISO processes
It is well established that ISO 9001 is a process-based standard, and the General Requirements listed in clause 4.4 establish this clearly:
| 1. Determine the processes of the QMS |
| 2. The sequence and interaction of those processes |
| 3. Identify process risks and controls |
| 4. The criteria and methods used to ensure QMS processes are effective |
| 5. Ensure availability of resources and information to operate and monitor the processes |
| 6. Monitor and measure and analyze the processes |
| 7. Continually improve the processes |
The first four items are the focus while setting up your ISO QMS. The remaining three become central as you maintain the ISO QMS and use it to improve the organization.
| 1. Determine the processes of the QMS |
SET UP |
| 2. The sequence and interaction of those processes |
SET UP |
| 3. Identify process risks and controls |
MAINTAIN & IMPROVE |
| 4. The criteria and methods used to ensure QMS processes are effective |
MAINTAIN & IMPROVE |
| 5. Ensure availability of resources and information to operate and monitor the processes |
MAINTAIN & IMPROVE |
| 6. Monitor and measure and analyze the processes |
MAINTAIN & IMPROVE |
| 7. Continually improve the processes |
MAINTAIN & IMPROVE |
Therefore, step one in creating the ISO 9001 QMS is to understand and define the processes of the QMS. Understanding your processes is the key to a functional QMS.
The million dollar question – what is the scope
There is a scope question each organization has to answer (which will also eventually be included in the Quality Manual).
Exactly what processes will be part of the ISO 9001 QMS?
The ISO 9001 QMS could encompass the entire organization, a particular facility, or perhaps just a single product line.
Our core business process – so what do we do every day?
One way to get started defining processes is to consider the over-arching, top level processes that convert supplies and resources (i.e., materials, knowledge, and capital equipment) into customer deliverables. Figure 3 shows an example of defined processes in an organization. If necessary, once top level processes are defined, you can identify key sub-processes that make up the top level process. Notice that Figure 3 also displays the interaction of the top level processes. Be sure to identify the process owners who have ultimate responsibility.
Creating the First Level of ISO 9001 Documents
Once the QMS processes are defined, you are ready to start the next step in phase 1: creating the first level of documentation. The first document tier in the QMS may consist of the Quality Manual, the Quality Policy, and the Quality Objectives.
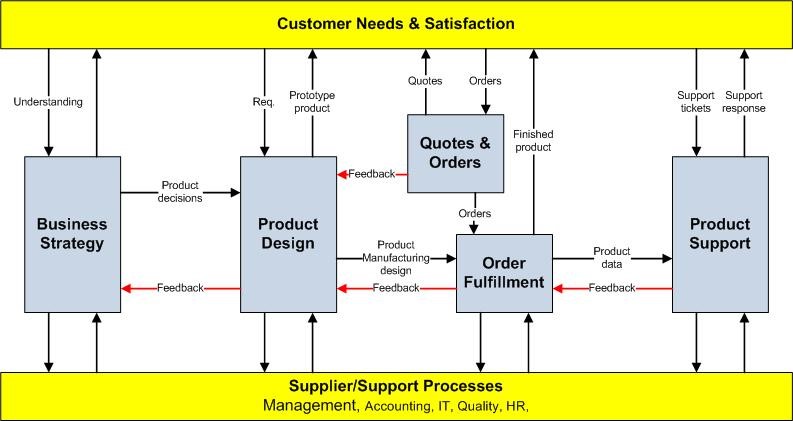
By having already clearly defined the QMS processes, creating a draft of the Quality Manual is easier since you have already determined much of the key content. Remember, the description and interaction of the processes in the Quality Manual does not have to be large amounts of text. Diagrams, process maps, and flow charts all describe processes just as well as, if not better, than written descriptions. Also, be sure to list all exclusions your organization has to the ISO 9001 QMS.
Exclusions – what can you leave out of ISO 9001
Exclusions are ISO 9001 requirements that do not apply to your organization. Sometimes you exclude minor requirements; sometimes you exclude large sections. For example, a contract manufacturer that does not do any design and development can exclude large parts of Clause 8. If you never have possession of customer property then you would want to exclude that clause. Failure to properly exclude or trying to over-exclude are common issues when developing an ISO 9001 QMS. Be sure to review exclusions carefully.
Completing Phase One with a Quality Policy, Objectives, and Document Control
At this point you have completed two crucial steps on your way to ISO compliance – defined processes and a draft of the Quality Manual. The next step is to write drafts of the quality policy and quality objectives. Remember, the quality objectives should be measurable and align/fulfill the quality policy. Just start with drafts for now, they don’t have to be perfect. But is it helpful to have a working draft of these key documents before you move forward.
The last step for phase one of your ISO QMS project is to create the documented information control system that will be used for the QMS documents. How is documented information reviewed and released, updated, stored, and retrieved? These questions should be addressed in ways that meet the requirements for documented information control established in Clauses 7.5.3.
Now, at the end of phase one, you have fulfilled most of the requirements established in Clause 4 of the ISO 9001 QMS Requirements, and you have also created the framework for meeting the additional requirements in other clauses of ISO 9001. While you may only have drafts of the quality manual and document and record control procedures, having a good draft in place represents a majority of the effort. Hopefully you will only need to make minor additions and revisions to them as you continue on your ISO 9001 QMS project path.
Editor’s note:
You are well on your way to implementing ISO 9001. If you have a document control procedure in your food safety system, you can use the same one. Now might be a good time to assess how easy it is to use and modify it if necessary. Remember keep it simple…